- Bullseye Trades
- Posts
- Insiders are loading up on this cancer play 🔥
Insiders are loading up on this cancer play 🔥
Its stock is far outrunning the big indexes
Sponsored Content on Behalf of MAIA Biotechnology*
The Santa Rally is in full swing, but one small pharma stock I’ve followed closely is dramatically outpacing the major indexes. With Roche and Regeneron partnerships in place, it’s up again in the pre-market this morning. Full details below.
TODAY’S TOP ALERT!
MAIA Biotechnology (NYSE: MAIA)
👉 MAIA is TODAY’S #1 ALERT* 👈
Happy Monday, Folks,
I hope you’re fully recovered from the Thanksgiving holiday because there’s serious momentum powering the markets right now.
The major indexes have ricocheted off their recent bottoms, and since Friday the 21st, the S&P 500 is up 4.7% and the Nasdaq is up 5.8%
The tech/AI pessimism appears to have melted away, and the “Santa Rally” is in full swing, though it appears to be taking a breather in early action today.
Right now, CME’s FedWatch tool is placing the odds of a rate cut next week at 87%.
A big thing to watch for is the September PCE inflation data which was delayed due to the government shutdown but is set to come out this Friday.
At the top of my watchlist today is a stock that has been dramatically outpacing the markets over the past week.
Since November 21, it’s up 35%.
I’ve alerted this stock no fewer than eleven times in 2025, and each time, it made double-digit intraday gains.*
It’s no surprise that Zacks Equity Research declared it one of the “Best Momentum Stocks to Buy” earlier this year.
Go to your favorite platform and pull up MAIA Biotechnology, Inc. (MAIA).*
On October 7, the company announced a crypto treasury strategy. Its board authorized “holdings of up to 90% of the Company’s liquid assets in various cryptocurrencies.”
After an initial uptick, the stock declined, possibly on potential dilution fears.
It had a brief rally in late October powered in part by a press release detailing “exceptional extended survival in third-line [non-small cell lung cancer] patients.”
MAIA CEO Vlad Vitoc said, “In addition, as of September 17, 2025, a patient that began therapy in March 2023 has shown survival of 30 months, or 912 days, an outstanding measure relative to many of the high-risk cancers.”
The stock was weighed down by the broader market pullback through November 21, but as I mentioned, it’s up 35% from there.
Company insiders have scooped up nearly 60,000 shares since that date:
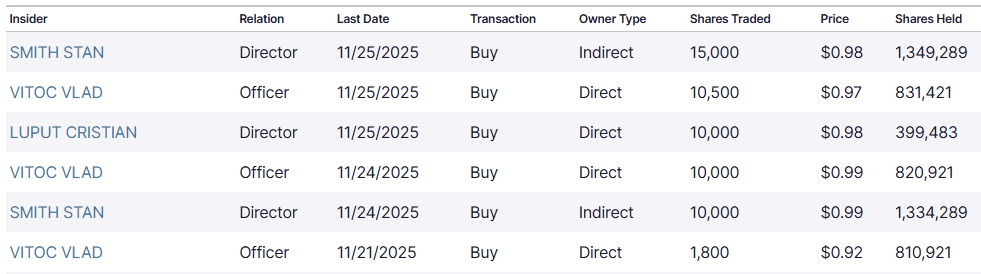
With clinical-stage biopharma companies like MAIA, it’s always worth paying close attention to what insiders are up to.
As of this writing, the stock is up again in the pre-market, so I’m watching it again today to see where the momentum takes it.
👉 MAIA is TODAY’S #1 ALERT 👈*
MAIA is a clinical-stage biopharmaceutical company exploring genuinely groundbreaking cancer treatments.
Here are some highlights you should consider as you do your own homework on the company right now…
1. A Potential Game-Changer in Cancer Treatment
MAIA’s flagship product, THIO (christened "Ateganosine" in March), is a potential game changer in oncology.
The company describes Ateganosine as “a potentially first-in-class small molecule that is the only direct telomere targeting agent currently in clinical development.”
Telomeres are like protective caps at the ends of chromosomes that cancer cells exploit to keep dividing endlessly.
Ateganosine breaks down these caps, triggering rapid cancer cell death while activating the immune system to keep fighting even after treatment stops.
This dual-action approach could revolutionize how we think about cancer therapies. Most existing treatments target the immune system or the cancer cells — not both.
MAIA announced in February its plans “to initiate a Phase 3 pivotal trial in 2025, named THIO-104, to evaluate the efficacy of THIO administered in sequence with a checkpoint inhibitor (CPI) in third-line non-small cell lung cancer (NSCLC).”
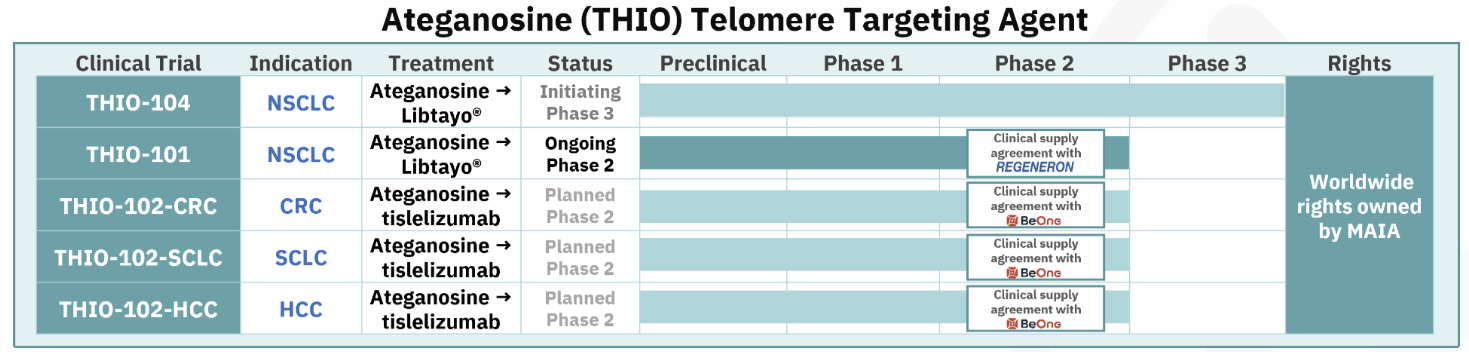
MAIA’s pipeline
On July 17, MAIA revealed the publication of preclinical data in a leading peer-reviewed journal, Nucleic Acids Research, demonstrating Ateganosine had “a significantly lower 50% inhibitory concentration with superior anticancer efficacy compared with the corresponding monotherapies.”
On August 27, the company said it had published developments in its Phase 2 THIO-101 clinical trial in a special issue of the journal Cells called “Cellular Mechanisms of Anti-Cancer Therapies.”
And on November 21, the company announced highlights from presentations at the Society for Immunotherapy of Cancer annual conference.
CEO Vlad Vitoc said that based on study results to date, “we believe that our Phase 3 trial could lead to an early commercial approval of ateganosine by the FDA.” [emphasis added]
2. FDA Orphan Drug and Fast Track Designations
In the biotech world, exclusivity is crucial. MAIA’s Ateganosine has Orphan Drug Designations from the FDA for small cell lung cancer (SCLC), hepatocellular carcinoma, and glioblastoma.
This would mean 7 years of market exclusivity post-approval plus tax credits for clinical trial costs.
On July 28, MAIA revealed:

CEO Vlad Vitoc noted that “If we are successful in the Fast Track regulatory pathway, ateganosine could qualify for accelerated FDA approval and robust exclusivity in NSCLC, with a potential FDA decision as early as next year.” [emphasis added]
For perspective, there are more than 226,000 new cases of lung cancer (SCLC + NSCLC) alone in the U.S. each year, and “Lung cancer is by far the leading cause of cancer death in the US, accounting for about 1 in 5 of all cancer deaths.”
If MAIA’s drug proves effective, it could dominate a potentially lucrative market.
3. Strong Early Data Is Turning Heads
Updated data from its THIO-101 Phase 2 trial — released on September 11 — were incredibly promising.
In a heavily pre-treated population, Ateganosine paired with Regeneron’s Libtayo achieved progression free survival (PFS) of 5.6 months — more than double the 2.5 months for the comparable standard-of-care.
MAIA’s CEO said the data demonstrate “the durability of ateganosine treatment through extended treatment cycles, which is in line with consistent tolerability and low toxicity.”
Results like these can attract big pharma partnerships and investor interest. Ateganosine is showing real potential to disrupt the market.
Also in September, the National Institutes of Health awarded MAIA a $2.3 million grant for the expansion of THIO-101.
They don’t hand those out to just anyone, folks.
MAIA’s senior medical director, Victor Zaporojan, called the grant “a tremendous achievement and a testament to the dedication, collaboration, and hard work of everyone involved in the clinical development of ateganosine.”
4. Partnership with Roche 🤝
On June 18, MAIA revealed a clinical master supply agreement with Roche (yes, $304 billion market cap Roche) “for future studies investigating the combination of MAIA’s telomere-targeting agent ateganosine (THIO), sequenced with Roche’s checkpoint inhibitor (CPI), atezolizumab (Tecentriq®), for the treatment of multiple hard-to-treat cancers.”
The two drugs were found to be “highly synergistic” in preclinical studies.
Between this and the Regeneron ($82 billion market cap) partnerships, MAIA’s Ateganosine has attracted attention from some serious pharma heavyweights. 🥊
5. Strong Insider Confidence 💼
Company insiders aren’t just talking, they’re buying MAIA shares hand over fist lately.
I mentioned the nearly 60,000 shares bought since November 21, but going back to November 2024, insiders combined have bought nearly 700,000 shares.
Why It Stands Out:
MAIA isn’t your typical biotech. With a truly novel approach to cancer, strong early data, and heavyweight partnerships, the company could be on the verge of something game-changing.
In August, Diamond Equity Research released an update note on MAIA highlighting “[r]ecent updates [that] further de-risk the THIO program in third-line [non-small cell lung cancer].”
Incorporating the company’s recent financials, Diamond revised its valuation of MAIA to $10.27 per share — 770% higher than Friday’s closing price.
Investors looking for exposure to next-gen cancer therapies should do their homework on MAIA immediately, beginning with the company website, as well as this September investor presentation and this March shareholder letter.
As always, be sure to approach your trading in a responsible manner. Trading is very risky, and nothing is ever guaranteed, so never trade with more than you can afford to lose.
Please read the full disclaimer at the bottom of this email as well so you are aware of our compensation and other conflicts of interest, as well as additional risks and considerations. Always have a well-thought-out game plan that takes your personal risk tolerance into consideration.
Bottom line: I’ve managed to alert MAIA on 11 occasions in 2025 when it went on to make double-digit gains the same day.*
It has bounced 35% since November 21, and with positive action in the pre-market, I’m watching it today to see where the momentum takes it.
Stay locked into MAIA for all the action!
To Your Success,

*DISCLAIMER: This entity is owned by Sherwood Ventures LLC (SV). To more fully understand any SV subscription, website, application or other service, please review our full disclaimer located at https://bullseyealerts.com/disclaimer/
Just so you know, what you're reading is curated content for which we have received a monetary fee (detailed below) to create and distribute. Let's be clear that investing can be quite the roller coaster as stock prices can have wild swings up and down, so consider those crucial risks before you ever consider trading anything we discuss. Make sure you check out our full disclosure down below for the details on how we were paid, the risks, and why these results aren't what you'd call “typical.”
Just a quick heads up about this ad you're reading—as we’ve said, even though we like the company referenced above, and all the facts we discussed above are true to the best of our knowledge, we are running a business here. To distribute this information and help offset the costs of maintaining our large digital audience, in advance of writing the content above, we currently have received twenty five thousand dollars (cash) from Sica Media for advertising MAIA Biotechnology, Inc for a one day marketing program starting on December 1, 2025. Previously. we have also received fifteen thousand dollars (cash) from Sica Media for advertising MAIA Biotechnology, Inc for a one day marketing program starting on October 27, 2025. We also received fifteen thousand dollars (cash) from Sica Media for advertising MAIA Biotechnology, Inc for a one day marketing program starting on October 23, 2025, and we also received twenty five thousand dollars (cash) from Sica Media for advertising MAIA Biotechnology, Inc for a one day marketing program starting on October 2, 2025. Before this, we received twenty five thousand dollars (cash) from Sica Media for advertising MAIA Biotechnology, Inc for a one day marketing program starting on September 11, 2025. Before this, we received thirty five thousand dollars (cash) from Sica Media for advertising MAIA Biotechnology, Inc for a one day marketing program starting on August 27, 2025. Prior to this, we received twenty thousand dollars (cash) from Sica Media for advertising MAIA Biotechnology, Inc for a one day marketing program starting on July 28, 2025. Additionally, we were paid twenty five thousand dollars (cash) from Sica Media for advertising MAIA Biotechnology, Inc for a one day marketing program starting on July 1, 2025. Before this, we received thirty five thousand dollars (cash) from Sica Media for advertising MAIA Biotechnology, Inc for a one day marketing program starting on June 18, 2025. Before this, we received twenty five thousand dollars (cash) from Sica Media for advertising MAIA Biotechnology, Inc for a two day marketing program starting on May 1, 2025. Before this, we received fifteen thousand dollars (cash) from Sica Media for advertising MAIA Biotechnology, Inc for a one day marketing program on April 23, 2025. Prior to that, we received fifteen thousand dollars (cash) from Sica Media for advertising MAIA Biotechnology, Inc for a one day marketing program on March 20, 2025. Before this, we received fifteen thousand dollars (cash) from Sica Media for advertising MAIA Biotechnology, Inc for a one day marketing program on February 27, 2025. Prior to that, we received thirty five thousand dollars (cash) from Sica Media for advertising MAIA Biotechnology, Inc for a one day marketing program on January 7, 2025, and we also received fifteen thousand dollars (cash) from Legends Media for advertising MAIA Biotechnology, Inc for a one day marketing program on December 16, 2024, and we received twenty-five thousand dollars (cash) from Sica Media for advertising MAIA Biotechnology, Inc for a one day marketing program on November 7, 2024, and also twenty-five thousand dollars (cash) from Sica Media for advertising MAIA Biotechnology, Inc for a one day marketing program on July 9, 2024 and also fifteen thousand dollars (cash) from Sica Media for advertising MAIA Biotechnology, Inc for a one day marketing program on June 13, 2024. Previously, we received twelve thousand five hundred dollars (cash) from Sica Media for advertising MAIA Biotechnology, Inc for a one day marketing program on June 7, 2024. Also, we were paid fifteen thousand dollars from Sica Media for advertising MAIA Biotechnology, Inc for a one day marketing program on March 6, 2024. We were also previously paid fourteen thousand dollars from Sica Media for advertising MAIA Biotechnology, Inc from a period beginning on November 15 through November 18, 2023, and also fifteen thousand dollars from Sica Media for advertising MAIA Biotechnology, Inc from a period beginning on October 12 through October 13, 2023. To date, we have received a total of four hundred sixty one thousand five hundred dollars for advertising MAIA Biotechnology, Inc.
These amounts were paid by someone else not connected to MAIA Biotechnology, Inc. It might be obvious, but whoever paid for this might own shares and is likely looking to sell some or all of them at any time after we send out this information, which might affect the stock price. We may also buy or sell shares in the company at some point in the future, although neither Sherwood Ventures nor its owners own any shares of the company at this time. Also, keep in mind that due to the sheer size of our audience, if even a small percentage of people decide they want to buy this stock, it could potentially boost interest enough to hike up those share prices and cause a temporary spike, and the opposite is possible as our program ends, though that is not always the case.
Now, diving right into MAIA Biotechnology, Inc might sound exciting. But remember, it’s like venturing into the wilderness—be aware that there's exceptional risk involved in trading. This isn't small potatoes we're talking about; you could lose every dime you put in, so always carefully think about what you’re doing. That’s why they call this trading, after all. We're shining a light on the good stuff about the company here, but it's on you to do your homework, make your own calls, and determine a plan for your own trading, hopefully with the help of your professional 1nvestment advis0r.
Oh, that brings us to another crucial point—we're not here to tell you (or even recommend) what you should do with your hard-earned money. We’re simply sharing our non-expert thoughts by highlighting some companies we like that could use some help telling their story to more people. We’re obviously biased in our writing. We’re not here to dig into anything that may be negative about the company; this is advertising, after all! Also, keep in mind that if we make some predictions about the future, these are technically known as “forward-L00king statements” under the securities acts, so take those with a grain of salt. As with all forecasts, they’re not set in stone, often wrong, and we certainly can’t know where the Company’s earnings, business, or share price will be tomorrow or a year from now.
Everything you read from us is all for your education, information, and possible entertainment. While we believe the info is reliable and accurate, we can't wear a cape and guarantee it. Before you jump into anything, make sure to talk it over with a pro—someone you trust who's licensed to give you real advice. To be clear, neither Sherwood Ventures nor its owners, employees, or independent contractors are registered as a secur1ties br0ker-dealer, br0ker, 1nvestment advis0r (IA), or IA rep’s with the SEC, any state securities regulat0ry authority, or any self-regulat0ry organization.
So, that's the scoop! If you're intrigued and want to learn more about the companies we talk about, hit up the SEC's website to dig into their filings and see the full picture.